 |
|
Overview The Okita laboratory has two major
research programs. One project centers on how RNAs are targeted to
specific subdomains of the cortical endoplasmic reticulum, the main
site of protein synthesis in plant cells. A second project is
directed towards understanding the structure-function of ADPglucose
pyrophosphorylase, a key enzyme of starch biosynthesis, at both the
biochemical and physiological levels. Additional information on these
research programs can be found below.
Although these studies appear to be totally unrelated, biochemical and
genetic studies indicate that carbon and nitrogen metabolism in the form
of starch and storage proteins are linked in storage organs. Hence, one goal of my
laboratory is to unravel the basis of this inter-relationship and to use
this information to manipulate these processes during development of
harvestable sink organs. |
|
|
RNAs on the Move Plant RNAs are transported to the cortical (peripheral) region for translation (Supported by grants from the National Science Foundation and USDA-NRICGP) Collaborators: professors Hikaru Satoh and Toshihiro Kumamaru, Kyushu University, Japan; Masahiro Ogawa, Yamaguchi Prefectural University, Japan; and Shigeki Hamada, Hokkaido University, Japan
|
Figure 1 Click on all pictures for larger versions |
|
RNAs are sorted to
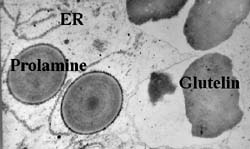
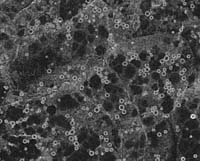
distinct ER subdomains Although prolamines and glutelins are synthesized on the ER, studies from my laboratory have demonstrated that these storage protein mRNAs are not randomly localized on the ER. Based on the RNA composition of subcellular fractions enriched for prolamine PBs and cisternal ER as well as from in situ hybridization analysis at the electron microscopy level of endosperm thin sections, prolamine RNAs are localized specifically to the surface of the prolamine protein body (PB-ER) while glutelin mRNAs are enriched on the cisternal ER membranes (Li et al. 1993 Cell 72:869-879). These ER membranes comprise the cortical ER membrane complex located near the plasma membrane. Figure 2 depicts the distribution of the spherical prolamine protein bodies in developing endosperm cells when viewed by immunofluorescence using BiP antibodies. Although BiP is a marker of the ER, this lumenal chaperone is not randomly distributed on the ER lumen but concentrated on the periphery of the prolamine intracisternal granule Li et al. 1993 Science 262:1054-1056. Because of this feature, we can identify the prolamine protein bodies as small ringlets (1-2 µm in diameter) when viewed by confocal microscopy (see Figure 2). Subsequent studies showed that these prolamine PBs were specifically labeled with the vital stain rhodamine hexyl ester. |
Figure 2
|
|
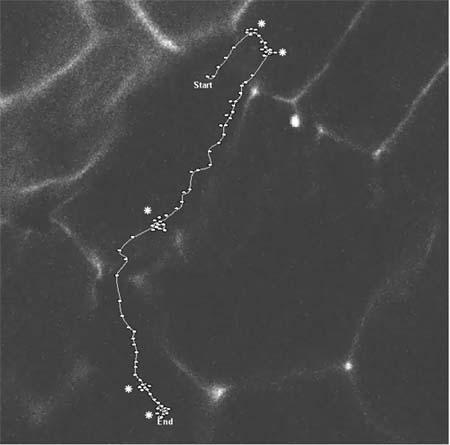
RNAs form large particles and move along actin filaments RNA movement can be visualized using non-evasive GFP technology developed by Robert Singer and his colleagues (see Bertrand et al., 1998 Mol. Cell. 2:437-445). Current studies indicate that prolamine and glutelin RNAs are transported as large macromolecular complexes ("particles") ranging in size from 0.1-0.5 μm in diameter (see Movie 1 and Movie 2). These particles move at an speeds up to 0.3 to 0.5 μm/sec although movement of the particle can stall or oscillate at a position before commencing movement again. The majority of the observed particles move directionally although bidirectional movement of some particles was detected. RNA movement is inhibited by cytochalasin and latrunculin B, indicating a role for actin filaments. Preliminary studies also show that prolamine RNAs are transported as particles to the peripheral (cortical) regions of tobacco BY-2 cells. If this observation is symptomatic of the plant condition then RNAs must be actively transported when they exited the nucleus to the cortical ER. |
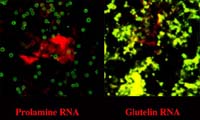
Figure 3
|
|
|
Bidirectional movement of prolamine RNA containing particles. In this movie clip, the RNA particle first moves into a NE direction. The particle begins to oscillate at two areas (designated by ä) before it changes directions. Each dot represents the location of the particle at 5-7 sec intervals. Note the particle travels somewhat out of the focal plane in the region before the third region of oscillation (ä).
|
|
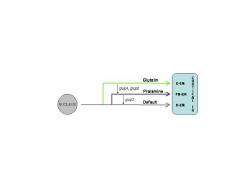
Three RNA transport pathways to the cortical ER Molecular and genetic studies have demonstrated that three RNA transport pathways exist; two regulated pathways and a default pathway. Deletion analysis of the rice prolamine and maize 10 kD zein RNAs showed that targeting to the PB-ER requires at least two cis-sequences or zip codes. When only a single zip code is present, the RNA is localized to both the PBs and cisternal-ER whereas the total absence results in RNA localization to the cisternal-ER (default pathway). Prolamine RNA has two zip code sequences while the 10 kD zein has four of these signal determinants. Glutelin RNAs are also transported by a regulated process as shown by the behavior of hybrid zein-glutelin RNAs. When the 3' untranslated region of the glutelin RNA is added to a zein RNA containing three zip codes, the hybrid RNA re-directed from the PB-ER to the cisternal-ER. These observations indicate that glutelin RNAs are transported to the cisternal-ER by a regulated process and that its cis-sequences are dominant over those found in rice and maize prolamine RNAs. The analysis of RNA targeting mutants in rice corroborates the existence of three RNA transport pathways. Three glup mutants, glup2, glup 4 and glup6, were identified based on their abnormal accumulation of glutelin precursor levels during seed development. In situ RT-PCR analysis indicates that glup4 and glup6 mis-directs glutelin RNAs to the PB-ER whereas glup2 mis-directs prolamine RNAs to the cisternal ER. These results together with those obtained by analysis of the PB-ER and cisternal-ER zip codes indicate that the three pathways are inter-related with the glutelin pathway showing dominance over the prolamine pathway which, in turn, is dominant over the default pathway.
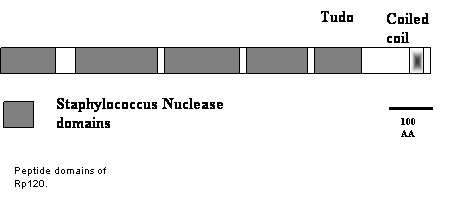
Rp120 - a multifunctional RNA binding protein and a component of the RNA transport particle A RNA binding activity, Rp120 (now called OsTSN), was identified in the enriched cytoskeleton-protein body fraction. OsTSN is a Tudor-SN homologue which has found to have multiple functions in the cell. In addition to being a transcriptional
co-activator and a major cytoplasmic and ER protein of lactating cells, Tudor-SN is a component of the RNA-induced silencing complex (RISC) and more recently found to specifically interact and degrade hyper-edited dsRNA. In rice OsTSN is found as two populations on sucrose density gradients; one that is soluble and a second form which is associated with dense macromolecular complexes which we believe to be the cytoskeleton. Indeed, indirect immunofluorescence studies shows that OsTSN coats microtubules and is present as small particles, some closely associated with prolamine PBs. Results from double immunofluorescence studies showed that OsTSN co-localize with GFP-tagged prolamine RNA transport particles, indicating a role in RNA localization.
Present studies on RNA localization underway Ongoing studies are: 1. Identification of glutelin zip codes responsible for re-directing prolamine zip codes from PB-ER to cisternal-ER. 2. Identification of RNA binding proteins responsible for RNA transport and localization. 3. Identification of loci in glup 2, glup4 and glup6 and interacting genes using a microarray approach. 4. Determine the RNA and protein composition of RNA transport particles. 5. Elucidate the role of OsTSN in RNA transport.
|
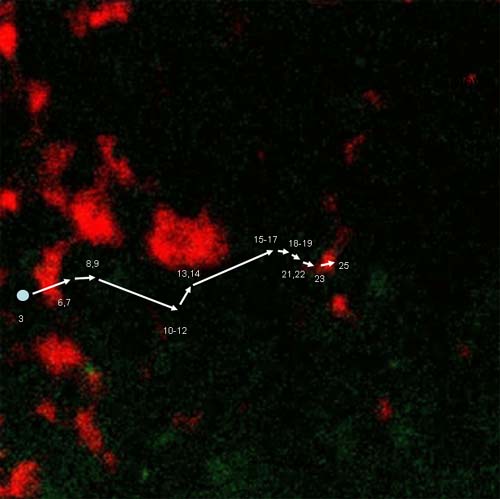
Movement of prolamine RNA particle to a prolamine
protein body (PBs). Prolamine
PBs were stained with Rhodamine hexyl ester. The numbers represent the time
frame where the particle is located.
Each frame was taken at 15 sec. intervals. Distance between start and final
destination is 21 μm.
|
|
Altering Source-Sink Relationships by Manipulation of ADP-glucose pyrophosphorylase (Supported by grants from the Department of Energy and USDA-NRICGP) Collaborators: Shigeki Hamada, Hiroyuki Ito and Hirokazu Matsui, Hokkaido University, Japan; Hikaru Satoh, Kyushu University, Japan; Jong-Sug Park, NIAB, RDA; Chul-Hee Kang, School of Molecular Biosciences, WSU. | |
| A
second major project in the laboratory is to elucidate the
structure-function relationships of ADP-glucose pyrophosphorylase (AGPase) a
key regulatory enzyme of starch biosynthesis and to use this information to
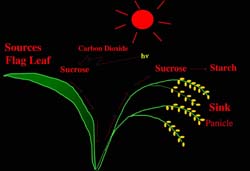
manipulate source-sink relationships in plants. Source-sink relationships, i.e. the capacity to photosynthesize and fix CO2 by source leaves and the capacity to assimilate this fixed carbon by sink tissues and organs, control the extent of plant productivity and crop yields (Figure 6). For many plants, overall productivity is limited by the sink's capacity to convert sugars into starch. In addition we have recently demonstrated that photosynthetic capacity and growth is correlated with the plant's ability to synthesize leaf starch. Hence, efforts in this laboratory are directed towards manipulating starch synthesis as a means of increasing the source-sink relationship.
|
Figure 6 |
|
The two AGPase subunits have different roles in enzyme function. Information derived from genetic and biochemical studies indicate that the LS and SS have different roles in enzyme catalysis and allosteric regulation. When expressed in the absence of the LS, the SS is capable of forming a catalytically active homotetrameric (α4) enzyme although exhibiting poor sensitivity to 3-PGA activation. Site-directed mutagenesis studies of the catalytic Asp and Lys residues of the putative 3-PGA binding sites by the Preiss laboratory confirmed the catalytic nature of the SS. The LS, when expressed alone, is unable to form an active enzyme or higher oligomeric structures and nearly all of the expressed protein is found in insoluble inclusion bodies (unpublished data). These observations suggest that the LS have mainly a regulatory function and when assembled with the SS renders the resulting heterotetrameric enzyme more sensitive to 3-PGA activation. Results from extensive analysis of AGPase enzymes formed from various mutant LSs and SSs, however, have provided a more detailed understanding on the roles of each subunit in enzyme function. Although the LS is required for optimal activation by 3-PGA and resistance to Pi, our results show that the regulatory and kinetic properties of AGPase are not simply due to the LS modulating the properties of the SS but, instead, are a product of synergistic interaction between the two subunits. The N- and C-terminal regions participate in allosteric regulation. N- and C-terminal peptide deletions on either the LS or SS have a major impact on the observed regulatory properties of the enzyme. Removal of 10 residues from the SS N-terminus renders the resulting heterotetrameric enzyme with increased affinity (2-fold) to the activator 3-PGA (as well as substrate Glc 1-P) and increased resistance (2-fold) to Pi. Likewise, removal of the corresponding peptide (17 residues) at the N-terminus of the LS had a similar up-regulatory effect in increasing the enzyme’s sensitivity to 3-PGA activation and Pi resistance. Removal of the putative carboxyl-terminal allosteric binding region from either subunit type resulted in an abolishment of enzyme formation indicating that the carboxyl-terminal region of each subunit type is essential for proper subunit folding and assembly. Combined, these results indicate that the N- and C-terminal regions are essential for the proper catalytic and allosteric regulatory properties of the potato AGPase. This view is supported by the identification and analysis of second site pseudo-revertants that restore an initial LS mutation which can be readily identified by the restoration of glycogen accumulation and iodine staining. Almost all of these dominant mutations were located in putative loop regions of the N- and C-termini. The role of the N-terminal region of the LS was further studied by site-directed mutagenesis of six Pro residues located in the first 66 residues. P66L displayed up-regulatory properties while P52L showed down-regulatory properties. Interestingly, the P44L enzyme showed only moderate changes in allosteric regulatory properties but, unlike the other mutants, displayed impaired catalytic properties. The structure of the LS N-terminal peptide has a pronounced effect on the AGPase catalytic and regulatory properties. Metabolic engineering of starch metabolism. In addition to defining the structure-function of AGPase at the biochemical level, a second long-term goal of this project is to apply knowledge obtained from our biochemical studies to increase plant productivity. In general, plant productivity is limited mainly by sink strength, i.e. the ability of sink organs such as developing seeds, roots, tubers etc., to metabolize photosynthate into dry matter. In addition to sink strength, productivity is also governed by processes that regulate photosynthesis in leaves especially when under sink-limited conditions. When sink tissues are unable to accommodate available sucrose, this metabolite builds up in leaf cells preventing Pi from being recycled. This ultimately leads to a reduction of Pi in the stroma which lowers photophosphorylation and the regeneration of ribulose bisphosphate and, in turn, net CO2 fixation. Recent findings indicate that leaf starch may play a much larger role than previously envisioned than simply serving a transient reserve of carbon and energy. It also serves as a transient sink accommodating excess photosynthate when sucrose levels are saturating. Such a condition would recycle Pi and would alleviate any potential feedback on photosynthesis. Current efforts are directed at manipulating leaf starch metabolism to alleviate photosynthesis feedback and seed starch metabolism to increase sink strength. Studies in Arabidopsis. Transgenic TL-46 (starch deficient line) expressing wildtype and up-regulated LS genes were obtained. After backcrossing to TL-46 to eliminate somaclonal variation effects, homozygous lines were studied biochemically and physiologically. All transgenic lines showed higher AGPase activity and elevated leaf starch than parental TL-46. Moreover, the transgenic lines displayed higher CO2 assimilation rates and O2 sensitivity due to an increased potential to utilize starch as a transient sink than wildtype plants. Collectively, these properties translated to an increase in growth and seed biomass in these plants compared to wildtype. Overall, these results indicate that leaf starch plays a significant role in modulating photosynthetic capacity. Studies in rice – Manipulation of leaf starch metabolism. Three rice lines transformed with the potato LUpReg1 gene under the control of the Rubisco small subunit promoter were identified which exhibited elevated leaf starch levels as indicated by the extent of iodine-staining during normal growth. Because of the limitations in growth facilities for optimum rice growth in Pullman, these lines were grown in field plots at the RDA, Suweon, Korea, a study in collaboration with Dr. Jong-Sug Park (see figure). Growth and yield measurements showed that the transgenic rice plants (at least 30 plants per line) exhibited significantly higher productivity (panicle length, number of panicles and number of spikelets) and yields than wildtype plants. Although the impact on photosynthetic capacity (CO2 assimilation rates, photosynthetic O2 evolution rates, and O2 sensitivity) remains to be assessed, these results support the view that increases in leaf starch provide, at the minimum, additional carbon and energy reserves during the night, which increase plant growth and development. Manipulation of rice seed starch metabolism. We introduced and expressed the E. coli glgC Triple Mutant (TM) gene, which encodes a highly active and allosterically insensitive AGPase, was introduced into rice and expressed during endosperm development. The mutant enzyme was targeted to either the amyloplast or cytoplasm to determine the relationship between intracellular location of ADP-glucose formation and starch synthesis. Transgenic rice seeds expressing the amyloplast or cytoplasmic AGPase-TM showed up to 13-fold higher levels of AGPase activity compared to untransformed plants but only when assayed in the presence of Pi to suppress the endogenous activity. Plants having elevated cytoplasmic AGPase activity under Pi-inhibitory conditions showed an increase in 14C-sucrose labeling into starch and, in turn, increase (up to 11 %) in seed weight than wildtype. In contrast, transgenic plants expressing the amyloplast-targeted AGPase activity showed little to moderate increases in 14C-sucrose-labeling rates into starch and either a moderate increase in seed weight or, in several instances, a reduction in seed weight. These results demonstrate that AGPase is one of the limiting factors in governing the extent of starch synthesis an, in turn, grain weight, and that the intracellular location of AGPase has a marked effect on the capacity on this process. These results differ from those obtained from the Giroux laboratory who showed that expression of a maize LS mutant (Shrunken 2 Rev6) gene resulted in higher grain yields due to increase seed number. Interestingly, transgenic rice plants expressing the cytoplasmic glgC-TM and grown under elevated CO2 showed no further increases in grain weight than those grown under ambient CO2. These transgenic lines did produce more seed due to increases in tillering. Overall, these observations indicated that the AGPase catalyzed step no longer poses a constraint towards starch biosynthesis in these transgenic plants and that other processes govern carbon flow into starch. Present studies on starch project 1. Continue exploring the relationship between LS and SS by using both biochemical and genetic suppressor approaches. 2. Develop conditions for optimal crystals of the heterotetrameric enzyme for structural determination. 3. Evaluate transgenic plants modified for leaf starch and/or seed starch for changes in gene expression by microarrays and in metabolite levels.
|
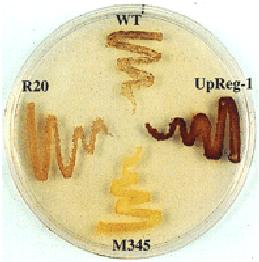 Figure 7
|
|
| |