Equipment: Gas proportional counter, pulse amplifier, pulse-height analyzer. Samples of elements and compounds. Gamma-ray source such as 241 Am or 57Co to excite x-rays.
Readings: Proportional counter, sect. 5.3 (especially 5.3.3). Know how to derive Moseley's law given by the equation above (consult an introductory modern physics text). Become familiar with the decay scheme and radiations of the gamma source you will use to excite x-rays (57Co (page 272) or241Am).
Key Concepts: Production of x-rays by photoelectric absorption.
K and L x-ray energies. Escape peak.

5.1 Energy calibration.
Keep counting rates low during calibration to avoid possible shifts when counting rates are high. Place sources some idstance from the proportional counter, with the counter shielded except for the (delicate) beryllium entrance window.
For 57Co, three peaks should be present in the pulse-height spectrum: the 6.4 keV K-xray of the daughter nucleus 57Fe, the 14.4 keV gamma ray of 57Fe, and a Rh or Pd x-ray, depending on the matrix in which the 57Co activity was diffused. For 241Am, photopeaks will be observed for 1 or 2 gamma-rays and for a bunch of Np daughter x-rays. Gamma-rays are at 59.54 keV and (harder to see) 26.3 keV. X-rays from the Np daughter are at 11.87, 13.93, 15.86, 17.61, and 21.00 keV. Other sources can also be used to provide calibration peaks.
Use these energies to calibrate the PHA and then adjust amplifier gain
so that energies up to, say, 100 keV can be stored in the PHA.
5.2 Characteristic K x-ray energies
The higher energy 59.5 or 122 keV gammas which follow decay of 241Am or 57Co, respectively, are absorbed in the target, followed to emission of characteristic x-rays. Both K or L x-rays will be produced, but the efficiency of the detector decreases at low and high energy so that both x-rays may not be detectable for a specified element (why?).
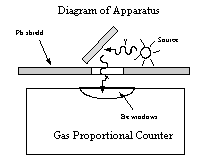
Direct an intense beam of gammas from the source onto a sample, but use a collimator and geometry as shown in the diagram to ensure that neither the beam nor scattering from objects other than the sample is detected. The x-ray flux from the sample will be low, so efforts to maximize the solid angle between target and counter window wil avoiding any direct beam from the gamma source will pay off in improved statistics. (see diagram below).
Measure characteristic x-ray energies for as many elements as you can.
To linearize the data, plot the square root of EK vs.
Z for known and unknown elements, and identify the unknowns. Indicate
experimental uncertainties on your plot. From the slope and intercept
determine the ionization energy of hydrogen, the screening constant
b. Estemate their uncertainties.
5.3 L x-ray energies
K x-rays of high Z elements like W and Pb may not be observable
(why?) and only L x-rays may be detected. Read about Moseley's extension
of his theory to explain L x-ray energies, and test it experimentally.
Questions and comments
a. What advantages does a gas proportional counter have over Geiger or scintillation counters for measurement of photon energies in the range 10-100 keV?
b. The method of x-ray production used here is nondestructive. Could the method be used for quantitative analysis of elemental composition? How? You might test this by analysis of the content of an alloy. For example, the nickle coin is reported to have a content of about 75% Cu and 25% Ni.
c. Different x-rays may be produced at the same time in a sample. Qualitatively, what determines the relative number of K and L xrays? How does the relative number depend on the energy of the gamma ray and on properties of the element?
d. If you used a 57Co source, you will observe that the Pd or Rh x-ray near 21 keV is much less intense than the Fe x-ray, although there are in fact very few Fe atoms in the source. Explain.
e. Nuclear decay via electron capture (e.g. in 57Co) leaves the atom in an excited state with, usually, one missing K or L-electron. Will deexcitation of the atom always lead to emission of an x-ray? If not, what are alternative processes? Beta-emission, on the other hand, is not obviously followed by x-ray emission. However, the pulse-height spectrum of 137Cs, for example, exhibits a large photopeak for Ba K-xrays. Why?
f. In pulse-height spectra, escape peaks are attributed to escape from the detector without detection of x-rays created during gamma absorption. X-ray escape is a more probable process in a gas counter than in a solid counter of similar size owing to its lower density. Most general purpose proportional counters are filled with Kr or Xe gas (typically plus a small amount of CO2 to quench the discharge). Under what conditions should escape peaks be more likely to be observed? Explain quantitatively if you can.
g. One might alternatively excite characteristic x-rays using bremmstrahlung
radiation from an x-ray tube. Very high fluences of exciting radiation
can be generated.. Characteristic xrays can be detected with better
energy resolution them using a semiconductor detector.